When You do not have continuous data it is not possible to calculate the Cpk, so how do You complete process validation?
I have previously written about sampling for capability and validation here, but capability is just one tool in the box for process validation.
Sometimes You do not have any actual measurements where you can calculate mean and standard deviation. Test methods where the output is Good/Bad or Yes/No cannot be used in a capability study as the data are discrete and not continuous. I.e. they are not numbers with decimals but 1’s and 0’s.
How do you provide evidence of Your process quality in that situation?
Well, if Your process is capable of producing good parts, then it is unlikely that you will find any defects in your sample. This fact is exactly what helps You provide the evidence You need.
If You do not find any defects at all in a sufficiently large sample, then Your process must be reliable. This is called zero defect sampling and in most situations it provides much stronger evidence for Your validation than the typical sampling standards intended for batch release.
So what is a sufficiently large sample?
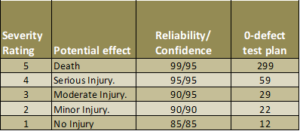
Most references will point to this table (or a very similar table) where the severity of a potential failure defines both how reliable Your process needs to be and how confident You need to be when you claim that reliability.
Notice that e.g. 90% reliability does not imply 10% un-reliable. It merely tells that You are not quite as confident about the remaining 10%.
The relation between sample size, confidence and reliability means that if You are 90% confident about a 90% reliability, then You are “only” 87% confident that the reliability is 91% and so on.
The fact that a process may cause reduced quality is not certain to end up as a hazard causing a harm to the end user.
Good luck with Your sampling……stay tuned for more